FDA poised to OK Pfizer’s coronavirus vaccine for emergency use, documents signal
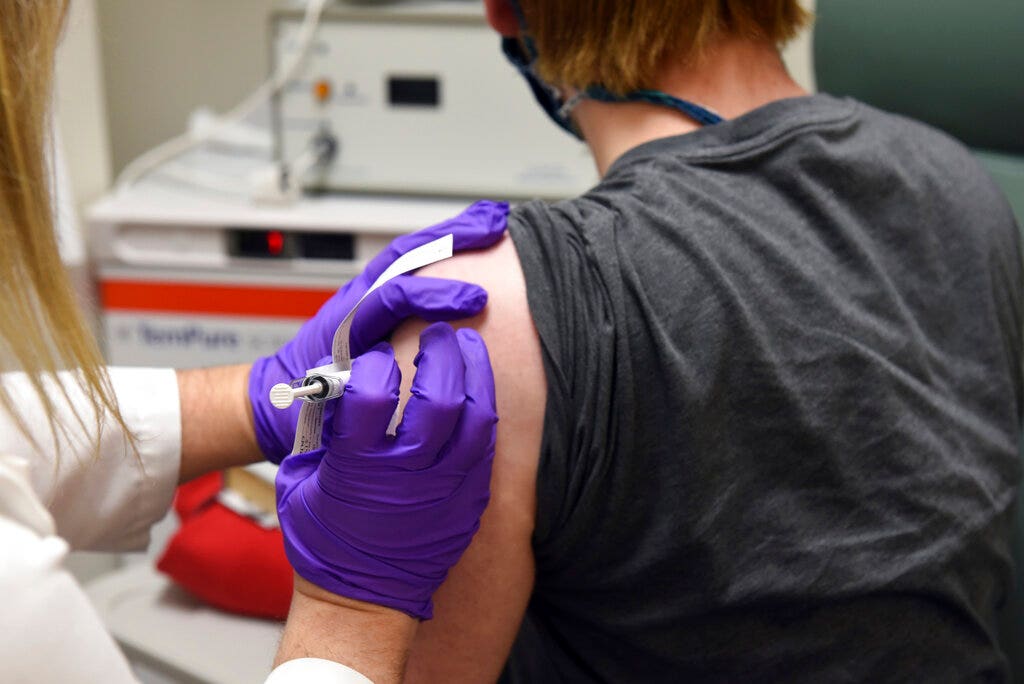
Pfizer and BioNTech’s coronavirus vaccine candidate meets the FDA’s requirements for emergency use authorization, according to documents posted ahead of the agency’s scheduled Dec. 10 meeting.
