FDA panel endorses Pfizer’s COVID-19 vaccine booster for people 65 and older, high-risk patients
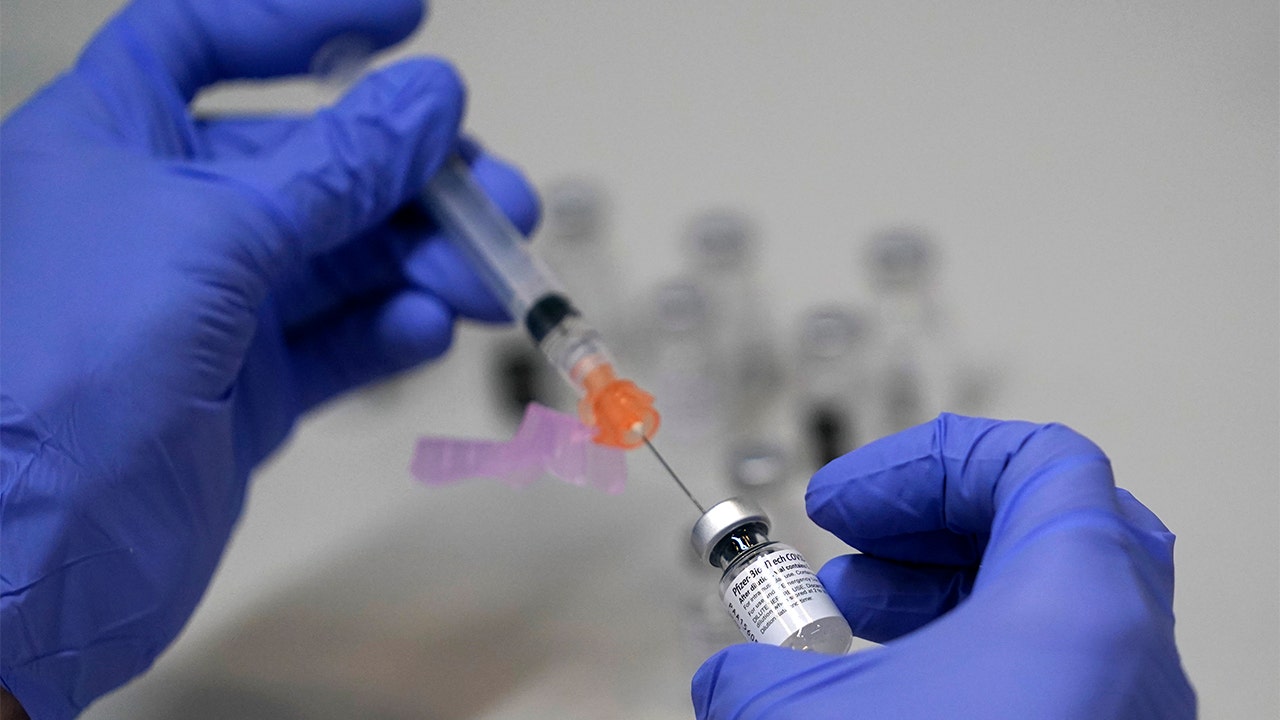
A U.S. Food and Drug Administration (FDA) advisory panel on Friday endorsed emergency approval for the Pfizer-BioNTech COVID-19 vaccine booster shot at least six months following the second dose among people ages 65 and older and those at high risk of occupational exposure and severe COVID-19.
